Difference between revisions of "Contrib/CompressibleMixingPhaseChangeFoam"
Mkraposhin (Talk | contribs) |
Mkraposhin (Talk | contribs) |
||
| Line 1: | Line 1: | ||
Solver for two fluids with phase change (for example - water <---> steam), pressure and temperature density dependence | Solver for two fluids with phase change (for example - water <---> steam), pressure and temperature density dependence | ||
| − | == Model Equations == | + | == Model Equations Derivation == |
* Equation of state | * Equation of state | ||
Low-compressible fluid: <math> | Low-compressible fluid: <math> | ||
| Line 147: | Line 147: | ||
-\frac{1}{\rho_1 C_{p,1}} \nabla \cdot \kappa_1 \nabla T -\frac{1}{\rho_2 C_{p,2}} \nabla \cdot \kappa_2 \nabla T | -\frac{1}{\rho_1 C_{p,1}} \nabla \cdot \kappa_1 \nabla T -\frac{1}{\rho_2 C_{p,2}} \nabla \cdot \kappa_2 \nabla T | ||
= | = | ||
| + | </math> | ||
| + | |||
| + | <math> | ||
\left ( \frac{\alpha_1}{\rho_1 C_{p,1}} + \frac{\alpha_2}{\rho_2 C_{p,2}} \right ) \frac{d p}{d t} | \left ( \frac{\alpha_1}{\rho_1 C_{p,1}} + \frac{\alpha_2}{\rho_2 C_{p,2}} \right ) \frac{d p}{d t} | ||
+ \frac{p}{\rho_1 C_{p,1}}\frac{d \alpha_1}{d t} + \frac{p}{\rho_2 C_{p,2}}\frac{d \alpha_2}{d t} | + \frac{p}{\rho_1 C_{p,1}}\frac{d \alpha_1}{d t} + \frac{p}{\rho_2 C_{p,2}}\frac{d \alpha_2}{d t} | ||
+ \dot m_1 p \left (-\frac{1}{\rho_1 \rho_1 C_{p,1}} + \frac{1}{\rho_2 \rho_2 C_{p,2}}\right ) | + \dot m_1 p \left (-\frac{1}{\rho_1 \rho_1 C_{p,1}} + \frac{1}{\rho_2 \rho_2 C_{p,2}}\right ) | ||
</math> | </math> | ||
| + | |||
| + | * Linking liquid volume transport to pressure equation is done by introducing <math>+\alpha_1 \nabla \cdot \textbf{U}</math> and <math>-\alpha_1 \nabla \cdot \textbf{U}</math> at r.h.s of volume fraction balance equation. Then, <math>-\nabla \cdot \textbf{U}</math> replaced by value from pressure equation | ||
| + | |||
| + | <math> | ||
| + | \frac {\partial \alpha_l}{\partial t} + \nabla \cdot | ||
| + | \left ( | ||
| + | \alpha_l \textbf{U} | ||
| + | \right ) | ||
| + | = | ||
| + | \alpha_1 \nabla \cdot \textbf{U} + | ||
| + | \dot m_1 \left (\frac{1}{\rho_1} \alpha_1 - \left (\frac{1}{\rho_1} - \frac{1}{\rho_2} \right ) \right ) + | ||
| + | </math> | ||
| + | |||
| + | <math> | ||
| + | \alpha_1 \alpha_2 \left ( -\frac{1}{\rho_1} \frac{d \hat \rho_1}{d t} + \frac{1}{\rho_2} \frac{d \hat \rho_2}{d t}\right ) | ||
| + | +\alpha_1 \alpha_2 p \left ( -\frac{1}{\rho_1} \frac{d \psi_1}{d t} + \frac{1}{\rho_2} \frac{d \psi_2}{d t}\right ) | ||
| + | +\alpha_1 \alpha_2 \frac{d p}{d t}\left ( -\frac{\psi_1}{\rho_1} + \frac{\psi_2}{\rho_2} \right ) | ||
| + | </math> | ||
| + | |||
* Phase change model | * Phase change model | ||
[http://www.os-cfd.ru/compressibleMixingPhaseChangeFoam/Solver.tgz Solver sources and tutorials located here] | [http://www.os-cfd.ru/compressibleMixingPhaseChangeFoam/Solver.tgz Solver sources and tutorials located here] | ||
Revision as of 17:21, 30 December 2012
Solver for two fluids with phase change (for example - water <---> steam), pressure and temperature density dependence
Model Equations Derivation
- Equation of state
Low-compressible fluid: 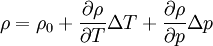
Ideal gas: 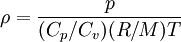
By combining this equations, we can get general relation:
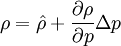
where 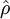 computed with respect to previous formulations
computed with respect to previous formulations
mixture density 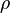 calculated as
calculated as
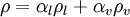
Here, and afterthere indices:
1,l,f - for liquid (heavy media with low compressibility)
2,g,s - for gas (light media (like steam) with big compressibility)
without index - mixture variable (or all variables local to some phase)
- Liquid volume transport
Let us consider transport of liquid (heavy phase) volume fraction 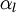 :
:
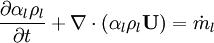
By converting to volume fluxes we get:
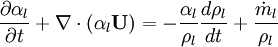
Using equation of state, we can reformulate substantial derivative for density in terms of pressure for any phase:
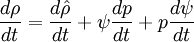
- General rule for converting from mass to volume fluxes in transport equation
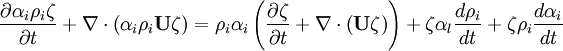
- Momentum equation (velocity prediction)
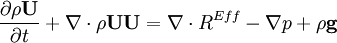 by substituting piezometric pressure
by substituting piezometric pressure 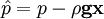 we get:
we get:
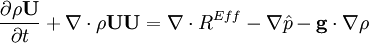
- Pressure equation obtained by summation of equation for volume phase fraction of liquid and gas phases:
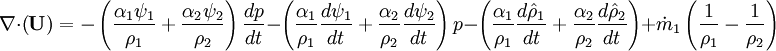
- Energy equation
Energy equation for mixture temperature obtained from sum of energy equations for each phase. Consider energy equation for phase-1:
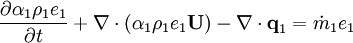
by converting to enthalpies we get:
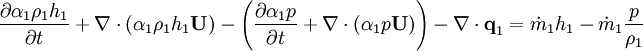
By substituting temperature instead of enthalpy, after conversion to volume fluxes we get equation for temperature (divided by
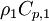 )
)
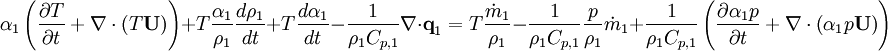
By combining equations of phases, we get energy balance for mixture:
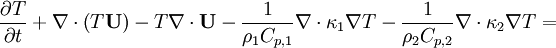
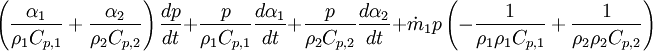
- Linking liquid volume transport to pressure equation is done by introducing
 and
and  at r.h.s of volume fraction balance equation. Then,
at r.h.s of volume fraction balance equation. Then, 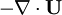 replaced by value from pressure equation
replaced by value from pressure equation
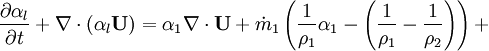
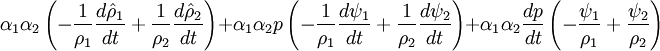
- Phase change model