Difference between revisions of "Contrib/CompressibleMixingPhaseChangeFoam"
Mkraposhin (Talk | contribs) (→Model Equations Derivation) |
Mkraposhin (Talk | contribs) (→Model Equations Derivation) |
||
| Line 107: | Line 107: | ||
* '''Energy equation''' | * '''Energy equation''' | ||
| − | Energy equation for mixture temperature obtained from sum of energy equations for each phase. Consider energy equation for phase-1: | + | Energy equation for mixture temperature obtained from sum of energy equations for each phase. Consider internal energy equation for phase-1: |
<math> | <math> | ||
| Line 113: | Line 113: | ||
\nabla \cdot \left ( \alpha_1 \rho_1 e_1 \textbf{U} \right ) + \nabla \cdot \textbf{q}_1 | \nabla \cdot \left ( \alpha_1 \rho_1 e_1 \textbf{U} \right ) + \nabla \cdot \textbf{q}_1 | ||
= | = | ||
| − | -\alpha_1 p \nabla \cdot \textbf{U} | + | -\alpha_1 p (\nabla \cdot \textbf{U} - \dot m_1 / \rho_1) |
+ | + | ||
\dot m_1 e_1 | \dot m_1 e_1 | ||
</math> | </math> | ||
| − | |||
| − | + | By combining equations of phases, we get internal energy balance for mixture in temperatures: | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | By combining equations of phases, we get energy balance for mixture: | + | |
<math> | <math> | ||
\frac{\partial T}{\partial t} + \nabla \cdot \left ( T \textbf{U} \right ) - T \nabla \cdot \textbf{U} | \frac{\partial T}{\partial t} + \nabla \cdot \left ( T \textbf{U} \right ) - T \nabla \cdot \textbf{U} | ||
| − | -\frac{1}{\rho_1 C_{ | + | -\frac{1}{\alpha_1 \rho_1 C_{v,1} + \alpha_2 \rho_2 C_{v,2}} (\nabla \cdot \kappa_1 \nabla T + \nabla \cdot \kappa_2 \nabla T) |
= | = | ||
</math> | </math> | ||
<math> | <math> | ||
| − | \left ( \frac{ | + | -\left ( \nabla \cdot \textbf{U} - \left(\frac{1}{\rho_1}-\frac{1}{\rho_2}\right) \dot m_1 \right ) |
| − | + | \frac{p}{\alpha_1 \rho_1 C_{v,1} + \alpha_2 \rho_2 C_{v,2}} | |
| − | + \ | + | - \left ( \frac{\partial L}{\partial t} + \nabla \cdot (\textbf{U} L) - L \nabla \cdot \textbf{U} \right ) |
| + | \frac{\alpha_2 \rho_2}{\alpha_1 \rho_1 C_{v,1} + \alpha_2 \rho_2 C_{v,2}} | ||
</math> | </math> | ||
| + | |||
| + | Where <math> L </math> - latent heat of evaporation | ||
* '''Linking liquid volume transport to pressure equation''' is done by introducing <math>+\alpha_1 \nabla \cdot \textbf{U}</math> and <math>-\alpha_1 \nabla \cdot \textbf{U}</math> at r.h.s of volume fraction balance equation. Then, <math>-\nabla \cdot \textbf{U}</math> replaced by value from pressure equation | * '''Linking liquid volume transport to pressure equation''' is done by introducing <math>+\alpha_1 \nabla \cdot \textbf{U}</math> and <math>-\alpha_1 \nabla \cdot \textbf{U}</math> at r.h.s of volume fraction balance equation. Then, <math>-\nabla \cdot \textbf{U}</math> replaced by value from pressure equation | ||
| Line 179: | Line 155: | ||
* Phase change model | * Phase change model | ||
| + | Schnerr-Sauer | ||
== Model Equations Summary == | == Model Equations Summary == | ||
Revision as of 15:31, 6 August 2013
Solver for two fluids with phase change (for example - water <---> steam), pressure and temperature density dependence
1 Model Equations Derivation
- Equation of state
Low-compressible fluid: 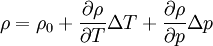
Ideal gas: 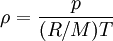
By combining this equations, we can get general relation:
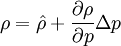
where 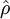 computed with respect to previous formulations
computed with respect to previous formulations
mixture density 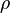 calculated as
calculated as
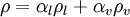
Here, and afterthere indices:
1,l,f - for liquid (heavy media with low compressibility)
2,g,s - for gas (light media (like steam) with big compressibility)
without index - mixture variable (or all variables local to some phase)
- Liquid volume transport
Let us consider transport of liquid (heavy phase) volume fraction 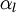 :
:
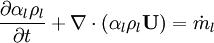
By converting to volume fluxes we get:
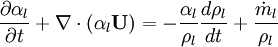
Using equation of state, we can reformulate substantial derivative for density in terms of pressure for any phase:
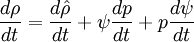
- General rule for converting from mass to volume fluxes in transport equation
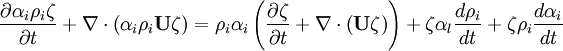
- Momentum equation (velocity prediction)
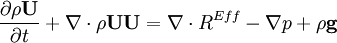
by substituting piezometric pressure 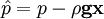 we get:
we get:
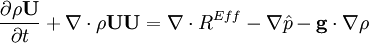
- Pressure equation obtained by summation of equation for volume phase fraction of liquid and gas phases:
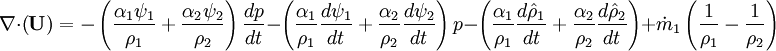
- Energy equation
Energy equation for mixture temperature obtained from sum of energy equations for each phase. Consider internal energy equation for phase-1:
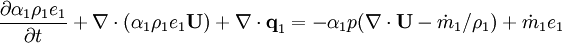
By combining equations of phases, we get internal energy balance for mixture in temperatures:
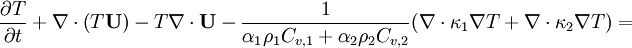
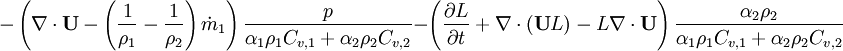
Where 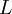 - latent heat of evaporation
- latent heat of evaporation
- Linking liquid volume transport to pressure equation is done by introducing
 and
and  at r.h.s of volume fraction balance equation. Then,
at r.h.s of volume fraction balance equation. Then, 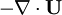 replaced by value from pressure equation
replaced by value from pressure equation
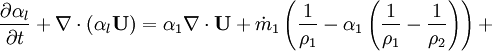
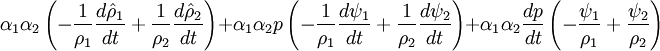
- Phase change model
Schnerr-Sauer
2 Model Equations Summary
3 Tutorial cases
- Case #1 - Water Evaporation in Cavity